News und Analysen

Pfizer and BioNTech Announce Collaboration With Brazil’s Eurofarma to Manufacture COVID-19 Vaccine Doses for Latin America
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced the signing of a letter of intent with Eurofarma Laboratórios SA, a Brazilian biopharmaceutical company, to manufacture

Pfizer and BioNTech Announce Collaboration With Brazil’s Eurofarma to Manufacture COVID-19 Vaccine Doses for Latin America
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced the signing of a letter of intent with Eurofarma Laboratórios SA, a Brazilian biopharmaceutical company, to manufacture

Pfizer and BioNTech Initiate Rolling Submission of Supplemental Biologics License Application to U.S. FDA for Booster Dose of COMIRNATY® in Individuals 16 and Older
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced the initiation of a supplemental Biologics License Application (sBLA) to the U.S. Food and Drug Administration (FDA) for the

Pfizer and BioNTech Initiate Rolling Submission of Supplemental Biologics License Application to U.S. FDA for Booster Dose of COMIRNATY® in Individuals 16 and Older
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced the initiation of a supplemental Biologics License Application (sBLA) to the U.S. Food and Drug Administration (FDA) for the

Clovis Oncology Announces Availability of and Reimbursement for Rubraca® (rucaparib) Tablets for Women with Relapsed Ovarian Cancer in Switzerland
Clovis Oncology, Inc. (NASDAQ: CLVS) today announced that Rubraca (rucaparib) is now available and reimbursed in Switzerland. The Swiss authority responsible for the authorization and supervision

Clovis Oncology gibt die Verfügbarkeit und Erstattung von Rubraca® (Rucaparib) Tabletten für Frauen mit rezidiviertem Eierstockkrebs in der Schweiz bekannt
Clovis Oncology, Inc. (NASDAQ: CLVS) gab heute bekannt, dass Rubraca (Rucaparib) nun auch in der Schweiz erhältlich ist und die Kosten erstattet werden. Die für die Zulassung und Überwachung von

Pfizer-BioNTech COVID-19 Vaccine COMIRNATY® Receives Full U.S. FDA Approval for Individuals 16 Years and Older
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced that the U.S. Food and Drug Administration (FDA) approved the Biologics License Application (BLA) for COMIRNATY® (COVID-19

Pfizer-BioNTech COVID-19 Vaccine COMIRNATY® Receives Full U.S. FDA Approval for Individuals 16 Years and Older
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced that the U.S. Food and Drug Administration (FDA) approved the Biologics License Application (BLA) for COMIRNATY® (COVID-19

Agilent Companion Diagnostic Expands CE-IVD Mark in Europe to Include Esophageal Cancer
Agilent Technologies Inc. (NYSE: A) today announced that the company’s PD-L1 IHC 22C3 pharmDx assay has expanded its use in Europe. The assay is now CE-IVD marked for use as an aid in identifying

Pfizer to Acquire Trillium Therapeutics Inc.
Pfizer Inc. (NYSE: PFE) and Trillium Therapeutics Inc. (NASDAQ/TSX: TRIL) today announced that the companies have entered into a definitive agreement under which Pfizer will acquire Trillium, a

Pfizer to Acquire Trillium Therapeutics Inc.
Pfizer Inc. (NYSE: PFE) and Trillium Therapeutics Inc. (NASDAQ/TSX: TRIL) today announced that the companies have entered into a definitive agreement under which Pfizer will acquire Trillium, a

XELJANZ® (tofacitinib citrate) Receives Marketing Authorization in the European Union for the Treatment of Active Polyarticular Juvenile Idiopathic Arthritis and Juvenile Psoriatic Arthritis
Pfizer Inc. (NYSE: PFE) announced today that the European Commission (EC) has approved XELJANZ® (tofacitinib) for the treatment of active polyarticular juvenile idiopathic arthritis (JIA) and

XELJANZ® (tofacitinib citrate) Receives Marketing Authorization in the European Union for the Treatment of Active Polyarticular Juvenile Idiopathic Arthritis and Juvenile Psoriatic Arthritis
Pfizer Inc. (NYSE: PFE) announced today that the European Commission (EC) has approved XELJANZ® (tofacitinib) for the treatment of active polyarticular juvenile idiopathic arthritis (JIA) and

Savara Announces New Employment Inducement Grant Under NASDAQ Listing Rule 5635(c)(4)
Savara Inc. (NASDAQ: SVRA), a clinical stage biopharmaceutical company focused on rare respiratory diseases, today announced the grant of inducement awards to two new employees.
On August 16

Simulations Plus Reports Continued Success in Second Phase of AIDD Collaboration with Large Pharmaceutical Company
Simulations Plus, Inc. (Nasdaq: SLP), a leading provider of modeling and simulation software and services for pharmaceutical safety and efficacy, today announced that it has received the second

Researchers Develop COVID-19 Severity Screening Method Based on the Agilent Cary 630 FTIR Spectrometer
Agilent Technologies Inc. (NYSE: A) announces that researchers at the Indian Institute of Technology Bombay in India and the QIMR Berghofer Medical Research Institute in Australia have developed a
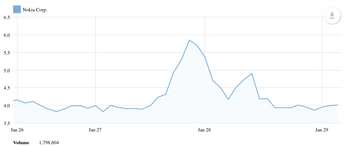
Agilent Reports Third-Quarter Fiscal Year 2021 Financial Results
Agilent Technologies Inc. (NYSE: A) today reported revenue of $1.59 billion for the third quarter ended July 31, 2021, an increase of 26% compared to the third quarter of 2020 and up 21% on a core(

Premier, Inc. Reports Fiscal-Year 2021 Fourth-Quarter and Full-Year Results and Issues Fiscal-Year 2022 Guidance
Premier, Inc. (NASDAQ: PINC) today reported financial results for the fiscal year 2021 fourth quarter and full year ended June 30, 2021 and issued its fiscal-year 2022 financial guidance.
"We are

Aurinia Acquires Novel Pipeline Assets Targeting Autoimmune and Kidney-related Diseases
Aurinia Pharmaceuticals Inc. (NASDAQ: AUPH) (Aurinia or the Company) today announced the addition of two novel assets that will expand the Company’s rare autoimmune and kidney-related disease

Pfizer Prices $1.0 Billion Sustainability Bond
Pfizer Inc. (NYSE: PFE) today announced the pricing of a sustainability bond of $1,000,000,000 in aggregate principal amount of 1.750% senior notes due 2031.
The closing of the offering is

Pfizer Prices $1.0 Billion Sustainability Bond
Pfizer Inc. (NYSE: PFE) today announced the pricing of a sustainability bond of $1,000,000,000 in aggregate principal amount of 1.750% senior notes due 2031.
The closing of the offering is

ChristianaCare Chooses Premier Inc. to Advance Supply Chain Innovation and Operational Excellence
ChristianaCare, one of the nation’s most dynamic healthcare providers headquartered in Wilmington, Delaware, has selected Premier Inc.’s (NASDAQ: PINC) industry-leading supply chain solutions, to

Clovis Oncology Announces Renewal of At-The-Market Equity Offering Program
Clovis Oncology, Inc. (NASDAQ:CLVS) announced today that it has filed a prospectus supplement with the U.S. Securities and Exchange Commission (“SEC”) to renew its previously established ATM

Pfizer and BioNTech Announce Submission of Initial Data to U.S. FDA to Support Booster Dose of COVID-19 Vaccine
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced that they have submitted Phase 1 data to the U.S. Food and Drug Administration (FDA) to support the evaluation of a third, or

Pfizer and BioNTech Announce Submission of Initial Data to U.S. FDA to Support Booster Dose of COVID-19 Vaccine
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced that they have submitted Phase 1 data to the U.S. Food and Drug Administration (FDA) to support the evaluation of a third, or





