News

Pharnext raises € 7.7 million in a private placement

Pharnext Announces Encouraging Data from Open-Label Phase 3 Extension Study of PXT3003 in Charcot-Marie-Tooth Disease Type 1A (CMT1A)

Pharnext establishes an equity line facility with Kepler Cheuvreux

EUROPLASMA: Drawdown ot the second tranche of 200 convertible bonds

Pharnext announces 2019 half-year results

DGAP-News: Biom'up announces the approval of HEMOBLAST(TM) Bellows and its laparoscopic applicator in Australia

Valbiotis invited to present clinical results at the American Diabetes Association’s 84th Scientific Sessions

Valbiotis reaches an agreement to terminate its licensing and supply agreement with Nestlé Health Science

Valbiotis launches its e-commerce site for the marketing of Valbiotis®PRO Cholestérol

Valbiotis publishes its annual accounts 2023

Valbiotis to launch its 100% natural dietary supplement for the management of hypercholesterolemia on the French market in May
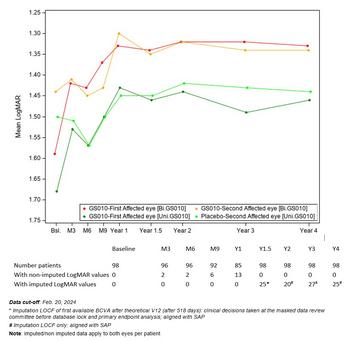
GenSight Biologics Confirms Sustained Efficacy and Safety of Bilateral LUMEVOQ® Injections Four Years After One-Time Administration
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240319984906/en/
Graph 1: Evolution of Best-Corrected Visual Acuity
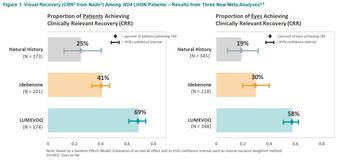
GenSight Biologics Announces Initial Results from New Meta-Analyses on Visual Outcomes with LUMEVOQ® Gene Therapy at NANOS 2024
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240311531952/en/
Figure 1. Visual Recovery (CRR from Nadir) Among
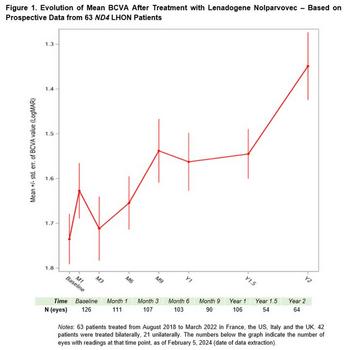
GenSight Biologics Announces Update on Real-World Data from Early Access Programs of LUMEVOQ® Gene Therapy at NANOS 2024
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240305810568/en/
(Graphic: Business Wire)
GenSight Biologics

Valbiotis presents its 2024 financial communication calendar

Valbiotis sets out its commercial and clinical roadmap for 2024

Valbiotis announces the success of its €15 M capital increase

Valbiotis announces the launch of a capital increase

Valbiotis announces the availability of an amendment to the Universal Registration Document

Valbiotis announces that it has received Food and Drug Administration (FDA) approval for New Dietary Ingredient (NDI) status

VALBIOTIS SA:

Valbiotis announces the success of the TOTUM•63 mode of action clinical study, against prediabetes and the early stages of type 2 diabetes

Median Technologies Appoints Ben McDonald to its Board of Directors
Median Technologies (Paris:ALMDT) announced today that Ben McDonald has joined its Board of Directors, chaired by Oran Muduroglu. This appointment was approved during the ordinary shareholders’

VALBIOTIS SA: the Phase II/III REVERSE-IT study selected for an oral presentation at the 2023 EASD congress, the main European learned society for diabetes






