News

Valbiotis publishes its financial report for the first half of 2023 and confirms its strategic roadmap

VALBIOTIS SA: Valbiotis presents the full results of the Phase II/III REVERSE-IT study: impressive efficacy of TOTUM•63 against prediabetes and the early stages of type 2 diabetes

Valbiotis announces its roadmap and strategic priorities on the eve of key milestones for its portfolio of innovative active substances

Valbiotis announces the appointment of Charlotte JEZEQUEL as Director of Human Relations and Executive Committee member
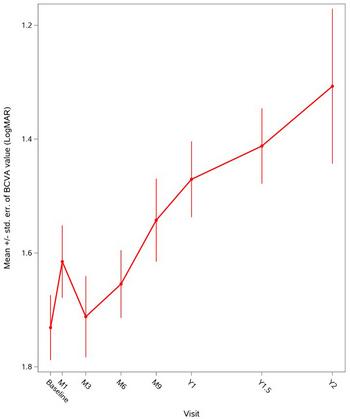
GenSight Biologics Announces Presentation of LUMEVOQ® Efficacy and Safety Data from Early Access Programs for ND4-LHON Patients at NANOS 2023
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230314006021/en/
Figure 1: Global evolution of mean BCVA over two
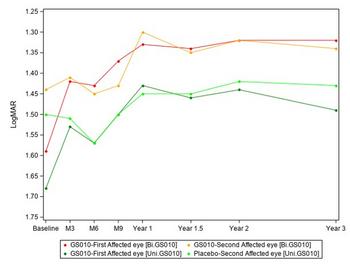
GenSight Biologics Confirms Sustained Efficacy and Safety of Bilateral LUMEVOQ® Injections at 3-Year Follow-Up of REFLECT Phase III Trial
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230312005028/en/
Graph 1: Evolution of Best-Corrected Visual Acuity
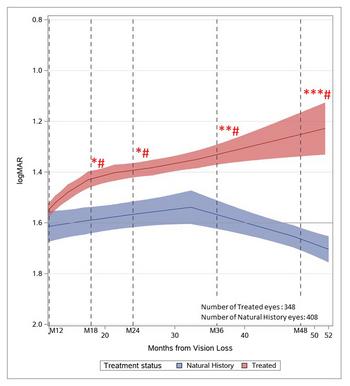
GenSight Biologics Announces Publication of Indirect Comparison of LUMEVOQ® Versus Natural History in ND4-LHON Patients in Peer-Reviewed Journal Ophthalmology and Therapy
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221214006010/en/
Figure 1: from Indirect Comparison of Lenadogene

GenSight Biologics reports 5 years’ data showing sustained efficacy and safety following one-time treatment with LUMEVOQ®
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220719006077/en/
Figure 1. Evolution of BCVA In LUMEVOQ®-treated

GenSight Biologics Reports Clinically Meaningful Vision Improvement is Maintained 4 Years After One-time Treatment with LUMEVOQ® Gene Therapy
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220123005091/en/
Figure 1. Evolution of BCVA In LUMEVOQ®-treated

GenSight Biologics Confirms Sustained Efficacy and Safety of Bilateral LUMEVOQ® Injections at 2-Year Follow-Up of REFLECT Phase III Trial
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20211213005948/en/
Figure 1. Best-Corrected Visual Acuity (BCVA) Change

BILENDI: Bilendi unveils 2 projects based on its BARI generative artificial intelligence, presented at the ESOMAR world congress

Sensorion Announces Its Participation in the 59th Annual Inner Ear Biology Workshop
Regulatory News:
Sensorion (FR0012596468 – ALSEN) a pioneering clinical-stage biotechnology company which specializes in the development of novel therapies to restore, treat and prevent within

Median Technologies to Attend and Present at the ESMO Congress 2024, Being Held in Barcelona, Spain, from Sept 13-17, 2024
Regulatory News:
Median Technologies (FR0011049824, ALMDT, PEA/SME eligible, “Median” or “The Company”) announces today that the Company will attend the European Society for Medical Oncology

Median Technologies to share positive pivotal data for eyonis™ LCS diagnostic software as medical device at the IASLC 2024 World Conference on Lung Cancer
Regulatory News:
Median Technologies (FR0011049824, ALMDT, PEA/SME eligible, “Median” or “The Company”) announces today that the Company will be sharing information on its proprietary

Median Technologies to host two webcasts on September 5, 2024
Regulatory News:
Median Technologies (FR0011049824, ALMDT, PEA/SME eligible, “Median” or “The Company”) will host two live webcasts on September 5, 2024.
Following the recent release of

Sensorion Announces its Participation in the World Congress of Audiology in September 2024 in Paris and will Lead a Symposium on Medical Advances in the Field of Hearing Loss
Regulatory News:
Sensorion (FR0012596468 – ALSEN) a pioneering clinical-stage biotechnology company which specializes in the development of novel therapies to restore, treat and prevent within

Median Technologies Announces Pivotal REALITY Study of Its eyonis™ LCS Lung Cancer Diagnostic Met All Primary and Secondary Endpoints
Regulatory News:
Median Technologies (FR0011049824, ALMDT, PEA/PME scheme eligible, “Median” or “The Company”) announces today that eyonis™ LCS, its proprietary Artificial Intelligence

Sensorion Announces its Participation in Stifel's Biotech Summer Summit
Regulatory News:
Sensorion (FR0012596468 – ALSEN) a pioneering clinical-stage biotechnology company which specializes in the development of novel therapies to restore, treat and prevent within

BILENDI: H1 2024 revenues: €30.6m, up +3.6%

Sensorion Announces Positive Recommendation From the Data Safety Monitoring Board (DSMB) Regarding the Continuation of NOTOXIS, its Phase 2a Clinical Trial of SENS-401 in Cisplatin-Induced Ototoxicity
Regulatory News:
Sensorion (FR0012596468 – ALSEN) a pioneering clinical-stage biotechnology company which specializes in the development of novel therapies to restore, treat and prevent within

GenSight Biologics Reports Cash Position as of June 30, 2024, and Provides Business Update
Regulatory News:
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal

ROCTOOL: First-half 2024 Revenue

Median Technologies Reports Record iCRO Backlog, eyonis™ LCS On-Track for Standalone Pivotal Study Readout in August
Regulatory News:
Median Technologies (FR0011049824, ALMDT, PEA/SME eligible, “Median” or “the Company”) reports today its business indicators for the first half of 2024, provide an update on

Sensorion Announces New Positive Secondary Efficacy Endpoints Data From SENS-401 Phase 2a Clinical Trial For The Preservation Of Residual Hearing Loss
Regulatory News:
Sensorion (FR0012596468 – ALSEN) a pioneering clinical-stage biotechnology company which specializes in the development of novel therapies to restore, treat and prevent within

GenSight Biologics Launches Newsletter for Retail Investors
Regulatory News:
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal





